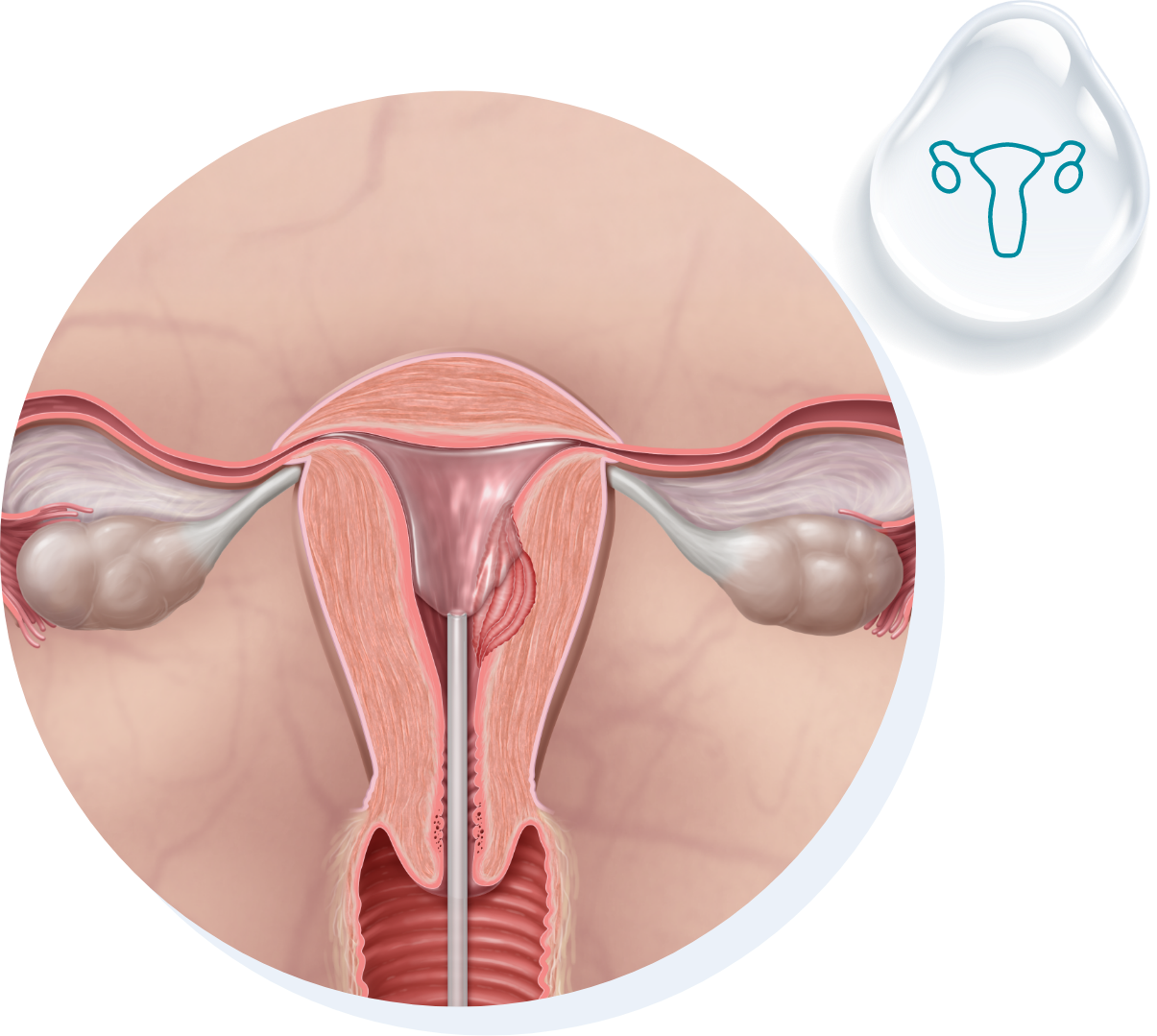
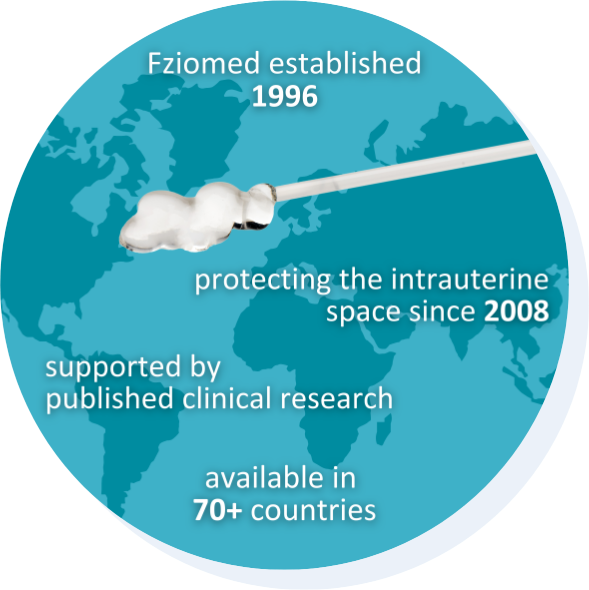
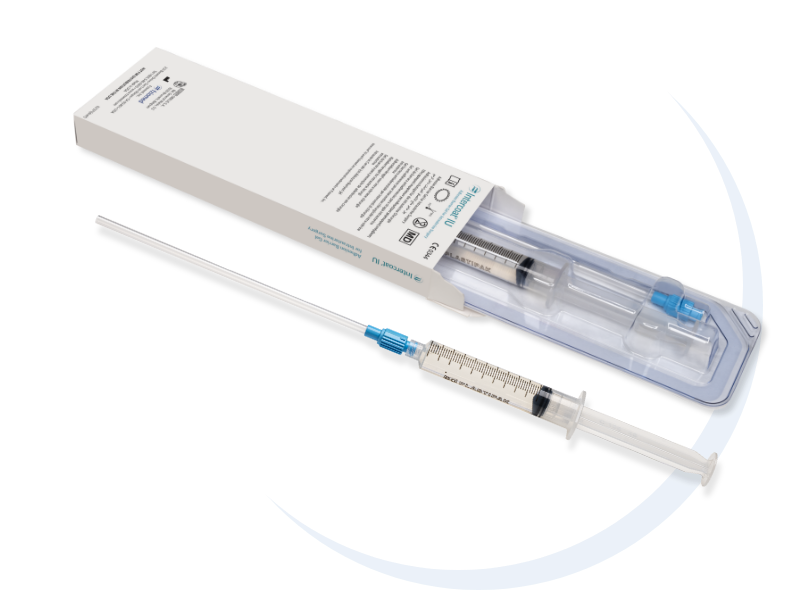
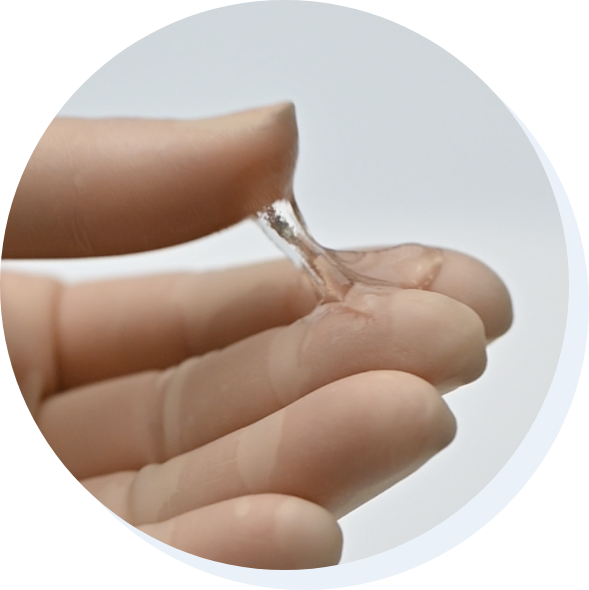
Fziomed, Inc.
231 Bonetti Drive
San Luis Obispo, CA 93401
USA
Phone: +1 (805) 546-0610
Fax: +1 (805) 546-0571
Email: [email protected]
Fziomed products are not currently available in the United States.
The following trademarks are owned by Fziomed, Inc. and registered in the United States and certain foreign jurisdictions: FZIOMED®, OXIPLEX®, OXIPLEX/SP®, DYNAVISC®, INTERPOSE®, INTERCOAT®, OXIPLEX/AP®, and OXIPLEX/IU®.
Website design RainCastle Communications.