Patented
Patented
Dual-Polymer
Technology
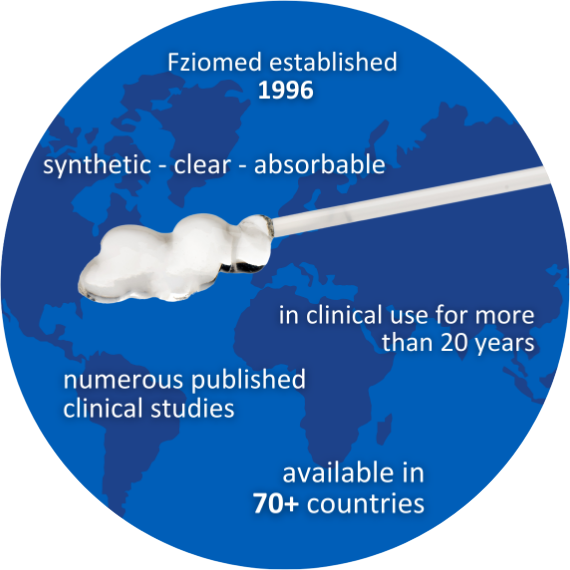
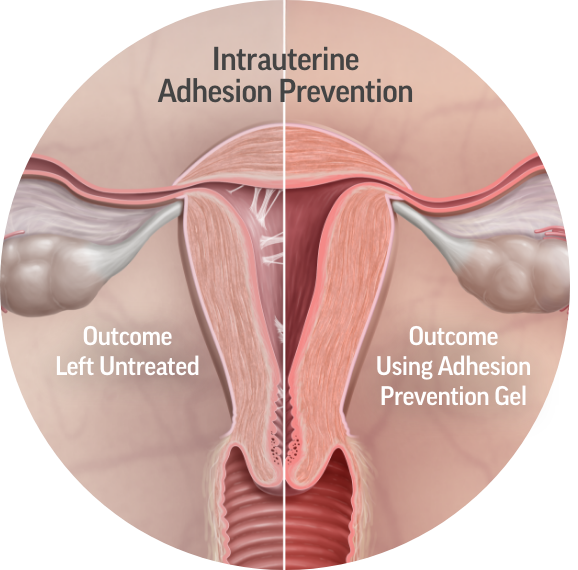
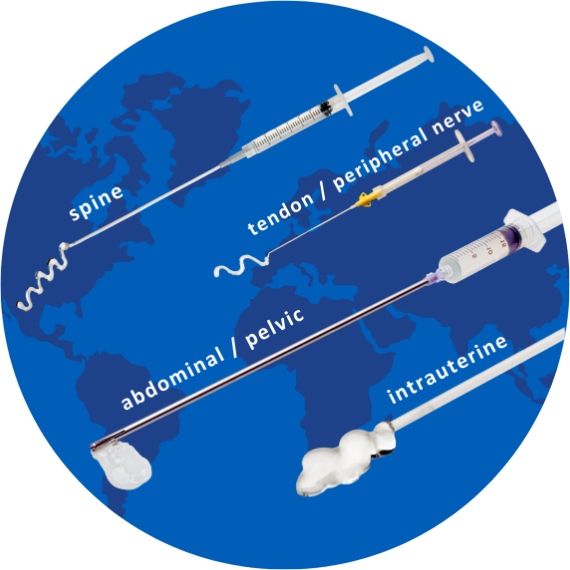
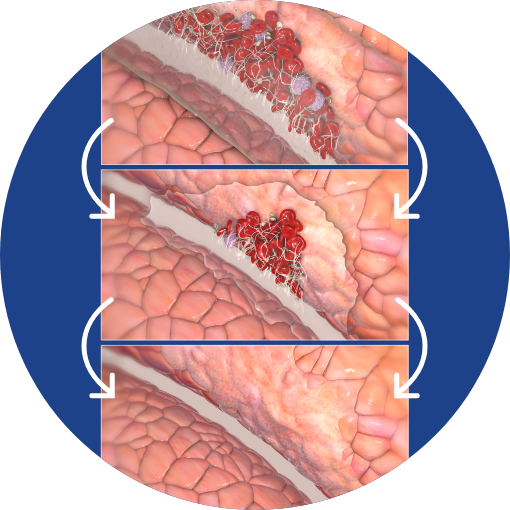
Our patented combination of two safe and extensively studied synthetic polymers—carboxymethylcellulose (CMC) and polyethylene oxide (PEO)—results in adhesion barriers offering a unique mode of action and combination of benefits.